The pharmaceuticals industry is at the forefront of a rapid digital transformation, embracing innovative technologies to streamline operations, enhance collaboration, and deliver life-saving drugs faster to the market. In this era of technological advancements, no-code, and low-code platforms have emerged as game-changers, enabling pharmaceutical companies to optimize their end-to-end operations efficiently.
These platforms empower citizen developers with domain expertise but limited technical backgrounds to create applications tailored to their specific needs without writing complex code. By infusing speed, agility, empowerment, and innovation into the hands of business users, no-code low-code in pharmaceuticals industry offers revolutionary solutions.
Embracing Citizen Development for Life Sciences
As the life sciences sector faces constant challenges and demands, the ability to innovate rapidly and streamline processes is critical. Citizen development is a transformative concept, empowering non-technical employees to actively participate in shaping digital solutions. Citizen developers become a strategic asset in the pharmaceutical industry, where domain expertise is indispensable. These individuals, equipped with a deep understanding of industry intricacies, can now directly contribute to building applications that address their unique requirements. This fosters faster problem-solving, heightened adaptability, and a stronger sense of ownership across the organization.
20 Transformative Use Cases for No-Code/Low-Code in Pharmaceuticals
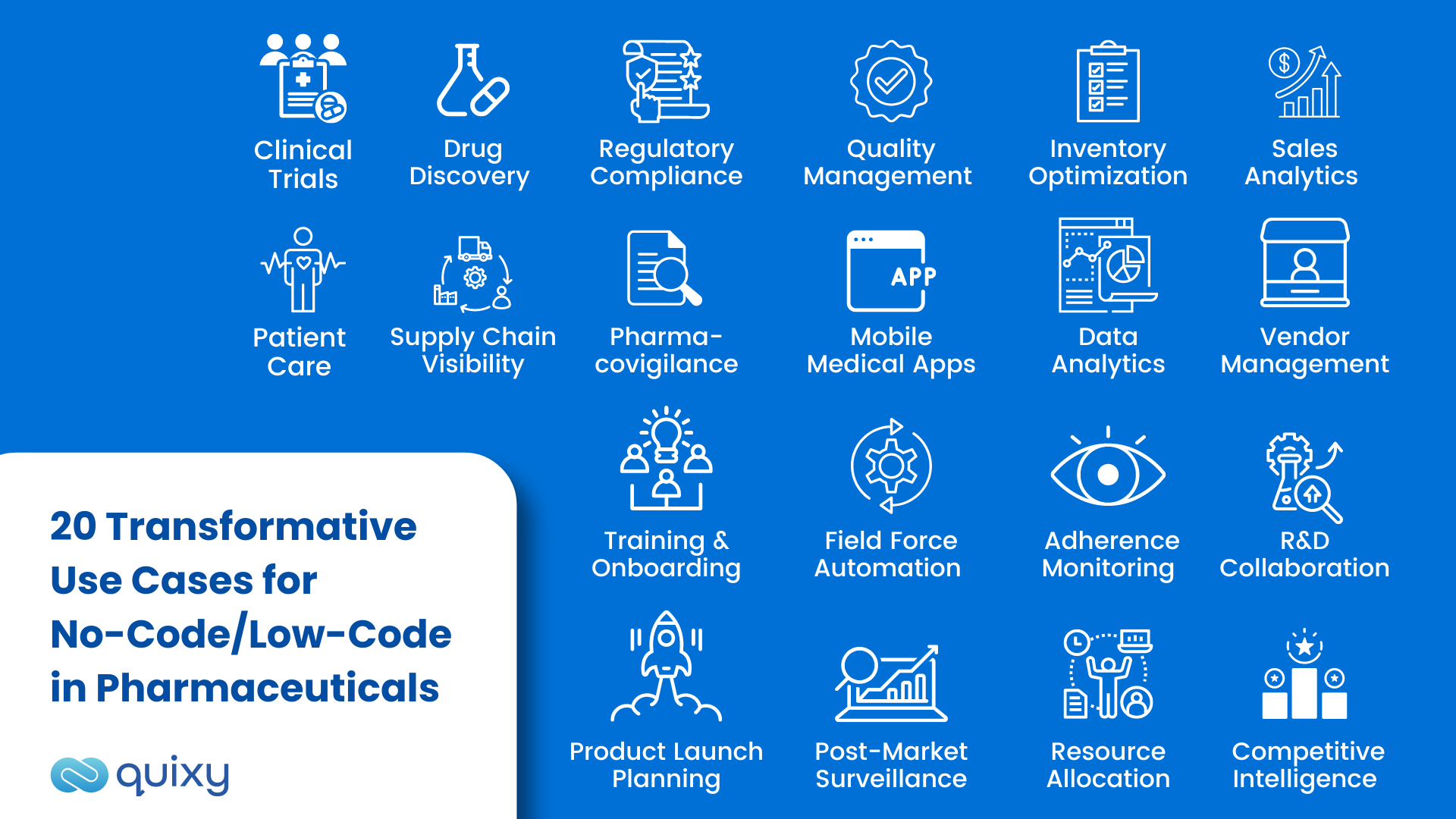
Clinical Trial Management
Streamline the entire clinical trial process with custom applications designed to track trial progress, patient recruitment, data management, and adverse event reporting. No-code automation ensures rapid data collection, seamless patient onboarding, and real-time insights. Automated processes and alerts and notifications keep stakeholders informed about critical milestones and protocol updates.
Drug Discovery and Research
Accelerate drug discovery efforts with applications that automate data analysis, identify potential drug candidates, and optimize research workflows. This leads to quicker identification of promising compounds. Real-time dashboards provide researchers with invaluable insights for data-driven decision-making.
Regulatory Compliance
Simplify regulatory compliance through automated tools for managing documentation, reporting adverse events, and adhering to industry guidelines. Seamlessly handle documentation and automate regulatory report submissions. Streamlined processes and timely notifications help compliance teams stay ahead of deadlines.
Quality Management Systems (QMS)
Create customized QMS applications to efficiently track quality deviations, CAPA (Corrective and Preventive Actions), and audits, ensuring strict adherence to quality standards. Automated processes manage quality deviations and CAPA processes, while real-time dashboards offer comprehensive visibility into quality metrics.

Inventory Management
Optimize inventory management by tracking stock levels and expiration dates and automating purchase orders. Automated processes ensure a seamless supply chain, while SMS and email alerts promptly notify stakeholders about low stock levels or expiring products.
Sales and Marketing Analytics
Empower sales and marketing teams with interactive dashboards, providing real-time insights for swift responses to market changes and maximizing opportunities. Automated reports and dashboards facilitate data-driven decision-making.
Patient Relationship Management
Enhance patient care and engagement through patient-centric applications that manage patient data, appointments, and interactions. Automated appointment reminders and follow-up processes improve patient adherence to treatment plans.
Supply Chain Visibility
Improve supply chain visibility with applications monitoring product shipments, forecasting demand, and managing supplier relationships. Stay informed about supply chain disruptions with automated alerts and notifications. Real-time dashboards offer insights into supply chain performance.
Pharmacovigilance
Automate pharmacovigilance processes for detecting and analyzing adverse drug reactions more efficiently, ensuring patient safety. Automated processes and alerts trigger rapid responses to product-related issues. Automated reports facilitate real-time pharmacovigilance monitoring.
Mobile Medical Apps
Design secure and user-friendly mobile medical apps for patient education, remote monitoring, and telemedicine using no-code/low-code platforms.
Also Read: No-Code for Business Departments to Empower and Streamline Operations across
Data Analytics and Business Intelligence
Empower teams with self-service analytics tools for data analysis and generating valuable insights without relying on IT. Automated reports and dashboards provide real-time visibility into critical business metrics.
Vendor Management
Develop applications to track vendor performance, compliance, and contract renewals, optimizing vendor relationships. Streamline vendor evaluation and contract renewal processes with automated processes and notifications.
Training and Onboarding
Facilitate employee training and onboarding through customized learning management systems and interactive modules. Automated processes manage training assignments and track completion progress.
Field Force Automation
Enable pharmaceutical sales representatives with mobile apps to manage customer interactions, product information, and sales data. Automate lead follow-ups and customer interactions for enhanced sales efficiency.
Adherence Monitoring
Track patient medication adherence to encourage better health outcomes and medication compliance. Create applications with automated medication reminders and healthcare appointment notifications.
R&D Collaboration
Promote cross-functional collaboration and knowledge-sharing through collaborative workspaces and document-sharing platforms. Automated processes manage document approvals and version control.
Product Launch Planning
Plan and execute successful product launches with applications coordinating marketing, sales, and supply chain activities. Automated processes ensure seamless launch planning and execution.
Post-Market Surveillance
Monitor product performance post-launch with automated applications for adverse event reporting and product feedback analysis. Automated processes facilitate signal detection and reporting.
Resource Allocation and Budgeting
Optimize resource allocation and budgeting processes with dynamic applications for financial planning and resource forecasting. Automated processes manage budget approvals and resource tracking.
Competitive Intelligence
Gather and analyze competitive intelligence with custom applications that track market trends, competitor activities, and pricing strategies. Automated data collection and analysis streamline competitive intelligence gathering.
Also Read: Empowering Future Business Leaders with the power of No-Code Citizen Development
Overall Benefits of No-Code/Low-Code and Citizen Development in Pharmaceuticals
- Rapid Application Development: Citizen developers rapidly build applications, reducing development time and accelerating time-to-value for digital solutions.
- Reduced IT Dependency: Empowering non-technical personnel reduces IT workload, allowing IT teams to focus on critical tasks and strategic initiatives.
- Enhanced Collaboration: Citizen development breaks down silos, fostering a cohesive working environment and promoting cross-functional collaboration.
- Innovation at Scale: Ideas for innovation can emerge from various organizational levels, fostering a culture of continuous improvement and creativity.
- Improved Compliance and Quality: Streamlined processes through no-code/low-code applications lead to better adherence to regulatory standards and higher product quality.
- Automated Workflows, Alerts, and Notifications: No-code/low-code solutions enable the implementation of automated processes, alerts, and notifications, improving operational efficiency and ensuring timely actions. Automated SMS and email notifications, reports, and dashboards facilitate seamless communication and data-driven decision-making.

Conclusion: No-Code Low-Code in the Pharmaceuticals Industry
Embracing no-code/low-code solutions and citizen development unlocks a world of opportunities for growth and innovation in the pharmaceutical industry. Rapid application development, streamlined processes, and enhanced collaboration empower organizations to achieve higher efficiency, comply with regulatory requirements, and spearhead progress. The simplicity and innovation of no-code/low-code transform how business users engage with technology, propelling the pharmaceutical industry into a future of efficiency and ingenuity. With citizen development leading the way, the industry is poised to thrive in an era of digital transformation and continued advancements.
Frequently Asked Questions (FAQs)
Q. How does no-code low-code enhance collaboration in pharma?
No-code low-code platforms provide a shared environment where research, development, and marketing teams can collaboratively build and optimize solutions. This encourages cross-functional communication and innovation within the organization.
Q. How to begin with no-code low-code in pharma?
First, identify specific pain points or areas for improvement in pharmaceutical processes. Explore reputable no-code/low-code platforms, assess their capabilities, and initiate small-scale projects to experience the transformative power firsthand.
Q. Can LCNC integrate with existing pharma systems?
Yes, many no-code/low-code platforms like Quixy provide integrations with common tools and databases used in the pharmaceutical industry. This allows for seamless connectivity between new solutions and existing infrastructure.
Q. What is the Impact of LCNC on pharmaceutical innovation?
No-code/low-code allows researchers to create custom data analysis, modeling, and simulation tools. This accelerates innovation by enabling rapid experimentation and insights extraction, facilitating faster progress in drug development.
Q. How do LCNC platforms benefit pharmaceutical companies?
These LCNC platforms empower pharmaceutical companies to swiftly develop customized applications, enhancing efficiency and reducing development time. From managing clinical trials to ensuring regulatory compliance, they streamline critical processes.
Login
Please login to comment
0 Comments
Oldest